US NEWS: Emergency Services Crews Often Unprepared for Diabetic Crises
By Serena Gordon
HealthDay Reporter
FRIDAY, Jan. 26, 2018 (HealthDay News) -- If you call 911, you expect to get the medical services you need.
But new research suggests that when it comes to severe low blood sugar episodes in people with diabetes, first responders might not be able to administer a potentially lifesaving medication called glucagon.
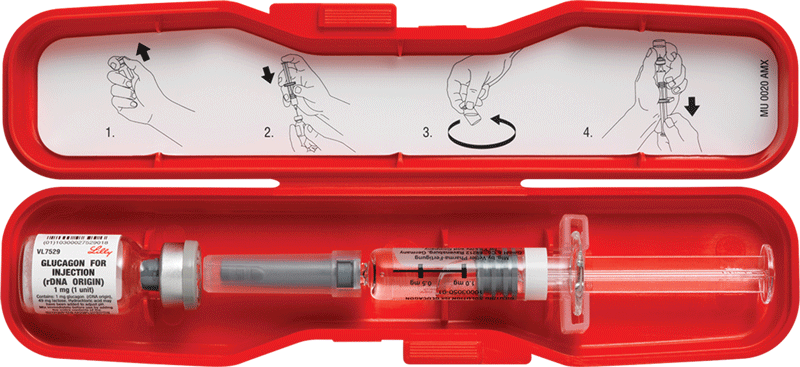
Glucagon is an injectable medication that prompts the liver to release stored glucose. This quickly raises blood sugar.
But paramedics can give the injections, said Dr. Craig Manifold, medical director of the National Association of Emergency Medical Technicians. That's because paramedics get between 750 and 1,500 hours of education compared to about 100 to 150 hours of training for EMTs.
Low blood sugar levels (hypoglycemia) generally occur in people with type 1 or type 2 diabetes taking insulin or other blood sugar-lowering medications. Researchers said more than 100,000 serious hypoglycemia episodes occur each year.
Gabbay noted even U.S. Supreme Court justices aren't immune to this problem. Earlier this month, Justice Sonia Sotomayor, who has type 1 diabetes, had to call emergency services for help with serious low blood sugar.
You can read the rest of the article here
Johnson & Johnson is trying to sell its diabetes care business
Johnson & Johnson is trying to sell its diabetes care business to Chinese buyers for up to $4 billion
- Johnson & Johnson, the world's largest health care company, is seeking to sell off its diabetes care businesses, including LifeScan, Animas, and Calibra.
- Several Chinese buyers are interested.
- The businesses could fetch up to $4 billion.
Chinese bidders are circling a diabetes care business owned by the world's largest healthcare company Johnson & Johnson in a deal that could fetch up to $4 billion, five people with direct knowledge told Reuters.
New Brunswick, N.J.-based J&J said in January last year it was evaluating options for its diabetes care companies, specifically LifeScan, Animas, and Calibra Medical. One option was a sale of the business, it said.
LifeScan makes devices to monitor blood glucose levels, which are key to controlling diabetes. Animas and Calibras -- which J&J acquired in 2012 -- make insulin delivery devices. In October, Animas, which makes insulin pumps, said it would shut its business in the United States and Canada amid increased competition and after failing to find a buyer.
Chinese interest in the J&J unit comes as the market for diabetes care in China is expected to grow rapidly. Almost one in three of the world's diabetes sufferers lives in China, according to World Health Organisation estimates.
Among the potential bidders is a consortium being formed by Shenzhen-listed Sinocare, which develops and manufactures blood sugar monitoring systems, and China Jianyin Investment (JIC), a unit of sovereign wealth fund China Investment Corp. The group has hired an advisor to work on a bid, according to two sources.
"The evaluation of potential strategic options for LifeScan and Calibra Medical Inc. is ongoing and we do not have an announcement regarding these businesses at this time," J&J said in a statement in response to Reuters request for comment.
The company has hired Goldman Sachs to work on the sale, according to three of the people. The bank declined to comment.
Sinocare's investors relations office said it could not confirm the information when contacted by Reuters. JIC and CIC did not respond to requests for comment. The sources declined to be identified.
Asia accounts for more than 60 percent of global diabetes cases, with increasing levels of wealth, unhealthy diets and more sedentary lifestyles sparking "diabetes epidemics" in the region, according to BMI Research.
George Lin, chief financial officer of Hua Medicine, a diabetes-focused drug developer, told Reuters on Wednesday that according to the most recent market research there were more than 110 million diabetes patients in China alone.
"The market right now in the world is already close to $50 billion," he said, referring to diabetes drugs. "In China, it is expected to grow from $6.6 billion in 2016 to $20 billion by 2025. This is a very large, fast-growing market."
Lin left a senior role at Bank of America Merrill Lynch to join Hua in December.
It is not yet clear if potential Chinese buyers are interested in the whole of J&J's diabetes care business or one or more of the member companies.
Sinocare, which has a market capitalisation of about $1.8 billion, in 2015 teamed up with Citic Securities to bid for Bayer's diabetes devices business that was eventually sold to Japan's Panasonic Healthcare Holdings, majority-owned by U.S. investment firm KKR.
JIC, wholly owned by CIC, mainly invests in the industrial manufacturing, consumption and information technology sectors, according to its website.
CIC's vice-chairman and president, Tu Guangshao, said at a panel discussion during the Asian Financial Forum in Hong Kong this week that it would look for more investment opportunities in the healthcare industry.
The sale of the diabetes business has also attracted interest from global private equity players, according to the people with knowledge of the process. But analysts said China could offer a tonic to J&J's struggling diabetes care unit and a turnaround opportunity for regional investors. Revenues at J&J's diabetes care unit have been falling since 2012, a Reuters study of the company's financial results found. In the first nine months of 2017, sales slid 7.7 percent year-on-year. In 2016, it suffered a similar decline.
Any sale by J&J of its diabetes device units would fit with a drive to exit from lower-margin, commoditized categories such as glucose meters and strips, but analysts said Asian buyers may be able to squeeze more out of the assets.
"Could a Chinese company extract more value from this than a multinational? It's possible because they have different expectations of profitability than multinationals so they can be happy with lower margins," said Franck Le Deu, Hong Kong-based senior partner at consultancy McKinsey.
"One complication of being in diabetes for a Chinese company is that you need a broad portfolio to be able to compete, and a broad footprint because it's a very dispersed market," he added. "So the investment levels needed to be competitive in diabetes are quite high, it's not an easy game to play."
Medicare Part D says yes to Omnipod!
Rejoice, revel, Read on!
The long and confusing winter has finally succumb to a common sense spring. No more shall Medicare mis-categorize our favorite tubeless insulin pump. I am thrilled to tell you that Omnipod is now covered by Medicare Part D! I also want to extend my sincere congratulations and excitement to everyone who has been waiting and working for this day. Medicare Part D coverage of Omnipod is one of the topics that I hear from you about most and I know from your messages that this has been a hardship for so many people.
I was speaking this morning with someone at Omnipod and they want you all to know just how much they appreciate the effort put forth by the diabetes community. Your support, persistence and patience did not go unnoticed.
“As you know, this has been a long, collaborative journey. The support of the diabetes community helped impress upon Congress and CMS the need for access to innovative methods of insulin delivery such as the Omnipod.”
MEDICARE PART D FAQ'S
How long did it take you to secure Medicare coverage and why did it take so long?
This has been a persistent effort since Insulet Corporation launched the Omnipod® Insulin Management System. The Centers for Medicare and Medicaid Services (CMS) communicated they had not comfortably assigned the Omnipod® System to a benefit category due to the unique design and physical attributes of the product. CMS has since issued guidance clarifying that products such as the Omnipod®System may be covered under the Medicare Part D (prescription) program. We are thrilled to now have the Omnipod® System formally covered by CMS as a Part D benefit.
What ultimately convinced CMS to provide coverage? Why did they change their mind?
It was less CMS changing their mind and more them clarifying that products such as the Omnipod® System fit into this criteria. CMS maintains that the Omnipod® System is not coverable as a DME benefit and therefore must be covered as a Part D benefit.
Does this Medicare coverage cover those living with insulin-dependent Type 2 diabetes?
We are assuming the Omnipod® System coverage criteria will be consistent with the coverage criteria outlined in Medicare’s Insulin Infusion Pump National Coverage decision which does not make any distinction between those individuals living T1 or T2 diabetes. While we believe there are opportunities to deliver the significant benefits of the Omnipod® System to the type 2 user base as we move forward, we are initially mainly focused on the T1D community for this coverage.
When will I be covered under Medicare Part D?
This decision enables your Part D carrier to add the Omnipod® System to their covered formulary list. Insulet Account Executives are now working with Part D carriers to ensure this occurs. This process takes time for the carrier to implement, so in the meantime our Medicare Access team may help you work with your Part D carrier to secure a formulary exception. Please feel free to connect with our Medicare Access team at 877-939-4384 t explore this process.
How wilI I know when my plan will cover the Omnipod® System?
Insulet will communicate updates to our progress working with Part D carriers to add the Omnipod® System to their formulary. Again, this process will take time. We should receive a decision from your carrier within several weeks about their decision to cover your supplies now via the formulary exception process. If you are a current Podder™, just click here to provide your insurance information online or reach out to our Medicare Access team at 877-939-4384 so we can help you initiate the formulary exception process.
How long could it take for my pharmacy to add the Omnipod® System to their formulary?
If the Omnipod® System is not on formulary for your plan today, we can assist you with the exception process. Once submitted, it will take about a week to know if the exception is approved. If not, we can pursue an appeal. In the meantime, we will also be working with your carrier directly to get the Omnipod® System put on the formulary for all of the carrier's members. It is difficult to predict how long it will take to get the Omnipod® System on formulary for your carrier a it could take months. Therefore the exception and appeal process is a good option if you want coverage sooner.
How do I find a Part D Medicare Plan that covers the Omnipod® System?
CMS’s decision to cover the Omnipod® System under the Medicare Part D program enables Insulet Corporation to begin contract negotiations with Medicare Part D carriers. Until these contract negotiations take place, the Omnipod® System is covered, but will not be listed as a covered product on the CMS Website. The good news is that both the Pod and Personal Diabetes Manager are available via the Part D formulary exception process. Please feel free to connect with our Medicare Access team at 877-939-4384 to begin the approval process.
How do I find a pharmacy that carries the Omnipod® System?
We currently have a number of pharmacies that are dispensing the Omnipod® System. Please call our Medicare Access team at 877-939-4384 to explore your pharmacy benefits and to learn what your Part D carrier's in-network pharmacy alternatives are.
How much will the Omnipod® System cost me?
The cost is dependent upon your Part D plan formulary benefits. We can begin a benefits investigation with you to determine what your cost will be. Please call our Medicare Access team at 877-939-4384 to begin exploring this.
How do I get started on the Omnipod® System?
Please call our Medicare Access team at 877-939-4384 and we can help you get started via the Part D formulary exception process.
What does my Medicare plan require me to do to get started on the Omnipod® System?
This will be dependent upon your Part D benefits. Please call our Medicare Access team at 877-939-4384 and we can work with you to begin the approval process.
This is not a sponsored post but Omnipod does support the Juicebox Podcast with ads. I wanted to be sure that you knew that. Speaking of support. If you are considering the Omnipod (and I think you should) please also consider using this link to try a free, no obligation demo. It helps the podcast when you click. Thanks!
Noninvasive BG monitoring with Apple Watch, "considered to be years away".
There is a recent article circulating in the T1 community that in part reads, "Citing anonymous sources within Apple, reports in Bloomberg and the New York Times over the past weeks continue to add to the pile of leaks and rumors about two of Apple's currently under-development health devices: a wrist-worn glucose sensor and an EKG built into the Apple Watch."
The article goes on to call the glucose sensor a, "a noninvasive continuous glucose reader".
If you were to surf over to the New York Times link on the subject and read down you would see this...
“Separately, Apple is continuing research on a noninvasive continuous glucose reader, according to two people with knowledge of the project. The technology is still considered to be years away, industry experts said.”
"considered to be years away..."
That's pretty much it. I don't want to be a Debbie Downer and I'm certainly not a Negative Nancy but I didn't want you all to feel like this was coming with the next version of the Apple Watch.
Now on to some good news...
I have a new giveaway for you to check out.
UCLA-led study blames mental lapses on sleep-deprived brain cells
Ever sleep poorly and then walk out of the house without your keys? Or space out while driving to work and nearly hit a stalled car?
A new study led by UCLA’s Dr. Itzhak Fried is the first to reveal how sleep deprivation disrupts brain cells’ ability to communicate with each other. Fried and his colleagues believe that disruption leads to temporary mental lapses that affect memory and visual perception. Their findings are published online today by Nature Medicine.
“Lack of sleep interfered with the neurons’ ability to encode information and translate visual input into conscious thought.”
“We discovered that starving the body of sleep also robs neurons of the ability to function properly,” said Fried, the study’s senior author, a professor of neurosurgery at the David Geffen School of Medicine at UCLA and Tel Aviv University. “This leads to cognitive lapses in how we perceive and react to the world around us.”
The remainder of the article is found here.